Some say that organic chemists these days can make any molecule. But can they be made easily? Our ideal is to develop practical, safe and environmentally benign synthetic methods where improved methods are needed. Naturally, we focus on methods that we need in our medicinal chemistry projects.
Copper-catalyzed N-arylation reactions
Copper is an abundant transition metal that can have excellent catalytic properties under the right conditions. We used copper in combination with a nucleophilic phenanthrolin ligand for the development of an efficient method for arylation of nitrogen in aqueous medium.
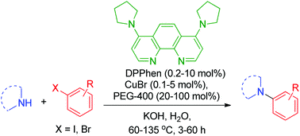
(Engel-Andreasen et al. Green Chem. 2012, 15, 336-340)
Purines make up an extremely important compound class, e.g. represented in two of the four nucleic acid bases. They are also ligands for several important receptors with caffeine probably as the most famous example. On this background, our method was developed further into the first efficient method for N-arylation of purines with aryl iodies.
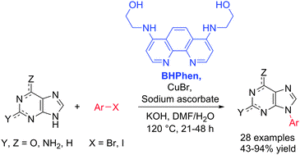
(Larsen & Ulven Chem. Commun. 2014, 50, 4997-5000)
Simple, safe and stoiciometric production of carbonmonoxide from oxalyl chloride
Carbon monoxide (CO) is a fabulous small building block, but its properties as a highly toxic odorless gas has made many chemists reluctant to bring a cylinder of CO into the lab. Our method makes pure CO in exactly the desired amount easily and cheaply available to anybody with standard equipment and a fume hood.
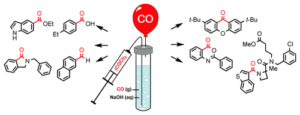
(Hansen & Ulven, Org. Lett. 2015, 17, 2832-2835)
Synthesis of difficult amides
Amides are everywhere since they link amino acids to form peptides and proteins, and synthesis of amides have often been considered a solved problem. However, most organic chemists have probalby encountered amides that they were unable to synthesize (even if they don’t talk about it…). Electron poor amines such as anilines can be quite difficult substrates for amide coupling, and often impossible if the substrates also are sterically hindered. We encountered one such amide in the synthesis of an FFA2 ligand. We solved the problem by converting the carboxylic acid to a minimally hindered acyl fluoride and performing the coupling with gentle heating. (The challenging nature of this particular amide was illustrated by its clean hydrolysis back to the starting material with only weak acid – in fact, since the FFA2 lignad also contained a carboxylic acid, it was unstable towards itself in neutral form.)
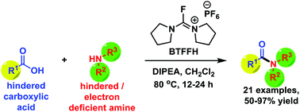
(Due-Hansen et al, Org. Biomol. Chem. 2016, 14, 430-433)
Improved Sonogashira coupling
Our first FFA1 series required assembly by Sonogashira coupling. Unfortunately, the unusually sluggish and unpredictable reactions with our substrate put a serious brake on the progress in the project. This was solved by adopting a ligand developed in the Beller group and performing the reaction under aqueous conditions.
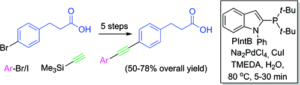
Synthesis of functionalized phenanthrolines
Phenanthrolines are efficient metal chelators that have a variety of used. We have used them as scaffolds in design of G-quaruplex stabilizing ligands and as ligands in transition metal catalyzed reactions.