Luminescent tools enable accurate real-time tracking of molecular entities in biological systems and have become ubiquitous in molecular biological research. We are especially interested in developing small-molecule fluorescent tools for studies of GPCRs. Ongoing project fall in two general categories: The first is tools for microscopy, where probes that emit long-wave light in the red or near-infrared bands are preferred for maximal tissue penetration and minimal interference from cellular autofluorescence. The second is tools designed to communicate with other molecules, such as tagged proteins. One way to do this is to tag the protein of interest (for us, typically a GPCR) with an enzyme that is able to convert a chemical substrate to light with a particular wavelength. If a fluorescent compound that is excited at the same wavelength comes nearby, the light is transferred from the enzyme to the probe in a process known as bioluminescence resonance energy transfer (BRET), and the probes emits light at a new wavelenght. For example, nanoluciferase (NLUC) consumes coelantrazine and produces light with 460 nm wavelenght.
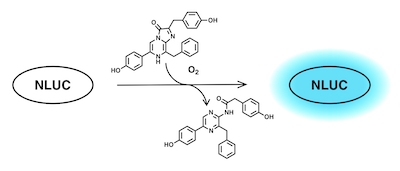
A fluorescent ligand that is excited at this wavelength will then shine in a different color when binding to a receptor that is tagged with NLUC. It turns out that the small fluorophore NBD (green star below) has an excitation band that overlaps almost perfectly with the emission band of NLUC.
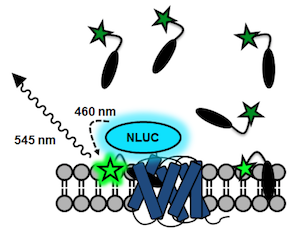
This is a principle that we have used for the generation of BRET-based binding assays for FFA1 and FFA2:
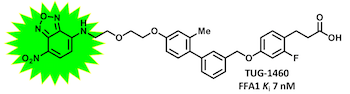
(Christiansen, Hudon, et al. J. Med. Chem. 2016, 59, 4849-4858)
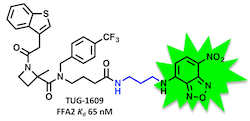
(Hansen, Sergeev, et al. J. Med. Chem. 2017, 60, 5638-5645)